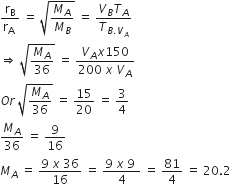pH of a saturated solution of Ba(OH)2 is 12. The value of solubility product Ksp of Ba(OH)2 is
-
3.3 x 10-7
-
5.0 x 10-7
-
4.0 x 10-6
-
5.0 x 10-6
B.
5.0 x 10-7
Given, pH of Ba(OH)2 = 12
pOH = 14-pH
= 14-12 = 2
We know that,
pOH = -log [OH-]
2 =-log [OH-]
[OH-] = antilog (-2)
[OH-] = 1 x 10-2
Ba(OH)2dissolves in water as