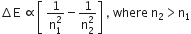Which has the maximum number of molecules among the following?
-
44 g CO2
-
48 g O3
-
8 g H2
-
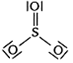
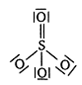
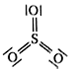
64 g SO2
A.
44 g CO2
44 g CO2 = 1 mol CO2 = NA molecules of CO2
48 g O3 = 1 mol O3 = NA molecules of O3
8 g H2 = 4 mol H2 = 4 x NA molecules of H2
64 g SO2 = 1 mol SO2 = NA molecules of SO2