A gaseous mixture was prepared by taking equal moles of CO and N2, If the total pressure of the mixture was found 1 atmosphere, the partial pressure of the nitrogen (N2) in the mixture is
-
0.8 atm
-
0.9 atm
-
1 atm
-
0.5 atm
C.
1 atm
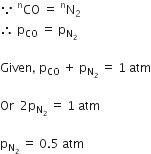
Sponsor Area
A gaseous mixture was prepared by taking equal moles of CO and N2, If the total pressure of the mixture was found 1 atmosphere, the partial pressure of the nitrogen (N2) in the mixture is
0.8 atm
0.9 atm
1 atm
0.5 atm
C.
1 atm
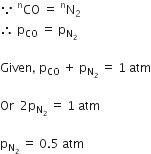
Sponsor Area
If x is the amount of adsorbate and m is the amount of adsorbent, which of the following relations is not related to adsorption process?
p = f(T) at constant (x/m)
C.
The correct relation is ![]()
Which of the two ions from the list given below have the geometry that is explained by the same hybridization of orbitals,




D.

Hybridization of the given molecule is 
therefore,  both have the same hybridization.
both have the same hybridization.
If the enthalpy change for the transition of liquid water to steam is 30kJ mol-1 at 27oC, the entropy change for the process would be.
1.0 J mol- K-1
0.1 J mol-1 K-1
100 J mol-1 K-1
10 J mol-1 K-1
C.
100 J mol-1 K-1
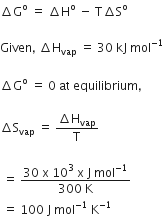
The Correct order of increasing bond length of C - H, C-O, C - C and C = C is
C - C < C=C < C - O < C - H
C - O < C - H < C - C < C = C
C - H < C - O < C - C < C= C
C - H < C = C < C - O < C - C
D.
C - H < C = C < C - O < C - C
C - H: 0.109 nm
C = C : 0.134 nm
C - O: 0.143 nm
C - C : 0.154 nm
Therefore, Bond length order is
C - H < C = C < C- O < C - C
Sponsor Area
Mock Test Series