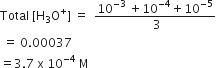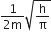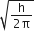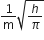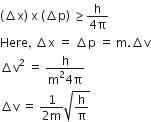Equal volumes of three acid solutions of pH 3.4 and 5 are mixed in a vessel. What will be the H+ ion concentration in the mixture?
-
1.11 x 10-4 M
-
3.7 x 10-4 M
-
3.7 x 10-3 M
-
1.11 x 10-3 M
B.
3.7 x 10-4 M
[H]+ in mixture
[H]+ of 1st acid x its volume + [H+]
of IInd acid x its volume + [H+] of 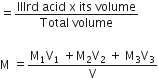
Assume the volume of each solution is 1 L.
[H3O+] in solution of pH = 3 is 10-3M
[H3O+] in solution of pH = 4 is 10-4 M
[H3O+] in solution is pH is 5 is 10-5 M