-
Call Us
+91 8076753736 -
Send us an Email
[email protected]
Structure Of Atom
Question
The value of Planck's constant is 6.63 x 10-34 Js. The speed of light is 3 x1017 nms-1 . Which value is closet to the wavelength in nanometer of a quantum of light with frequency of 6 x 1015 s-1 ?
-
10
-
25
-
50
-
75
Solution
C.
50
Given, Planck's constant,
h= 6.63 x10-34
speed of light, c= 3 x1017 nms-1
Frequency of quanta
v=6 x1015 s-1
Wavelength, λ =?
We know that,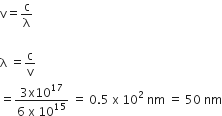
Sponsor Area
Some More Questions From Structure of Atom Chapter
What is a proton?
Who discovered neutron?
What is a neutron?
What is the name of the remaining part of atom except outer orbit?
Name the particles which determine the mass of an element.
What are α-particles?
Why were neutrons discovered very late?
What are the fundamental particles present in a neutral atom having atomic number greater than 1?
Do protons and neutrons have identical mass?
Mock Test Series
Sponsor Area
 Wired Faculty
Wired Faculty



