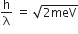A stream of electrons from a heated filament was passed between two charged plates kept at a potential difference V esu. If e and m are charge and mass of an electron, respectively, then the value of h/λ (where λ is wavelength associated with electron wave) is given by:
-
2meV
-

-

-
mev
C.

The relation between h/λ and energy is given as:
Applying de-Broglie wavelength and kinetic energy term in eV.
de-Broglie wavelength for an electron (λ) = h/p
⇒ p = h/ λ (i)
Kinetic energy of an electron = eV
As we know that,
From equations (i) and (ii), we get