The incorrect expression among the following is
-

-
In isothermal process
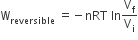
-

-

C.

Option C has incorrect expression. The correct expression is,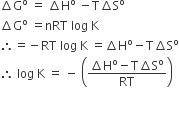
Sponsor Area
The incorrect expression among the following is

In isothermal process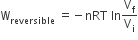


C.

Option C has incorrect expression. The correct expression is,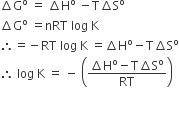
Sponsor Area
The species which can best serve as an initiator for the cationic polymerization is
LiAlH4
HNO3
AlCl3
BaLi
C.
AlCl3
Electron-deficient species (Lewis acid) like AlCl3, BF3, etc, are is used as an initiator for cationic polymerisation.
The standard reduction potentials for Zn2+/ Zn, Ni2+/ Ni, and F2+/ Fe are –0.76, –0.23 and –0.44 V respectively. The reaction X + Y2+ → X 2+ + Y will be spontaneous when
X = Ni, Y = Fe
X = Ni, Y = Zn
X =Fe, Y= Zn
X = Zn, Y = Ni
D.
X = Zn, Y = Ni
X = Zn, Y = Ni
Zn + Ni2+ →Zn2+ + Ni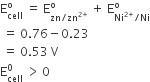
The equilibrium constant (Kc) for the reaction, N2(g) + O2 (g) → 2NO (g) at temperature T is 4 x 10-4. The value of Kc for the reaction 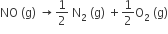 at the same temperature is
at the same temperature is
0.02
2.5 x 102
4 x 10-4
50.0
D.
50.0
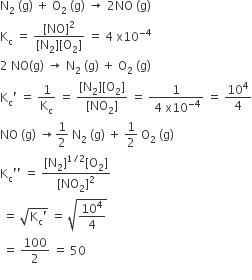
The compressibility factor for a real gas at high pressure is
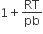

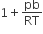
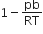
C.
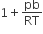
Vander Waal's equation for one mole of real gas is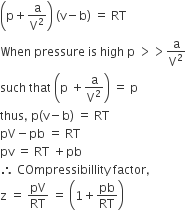
Sponsor Area
Mock Test Series