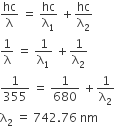Among the following, the maximum covalent character is shown by the compound
-
FeCl2
-
SnCl2
-
AlCl3
-
MgCl2
C.
AlCl3
The covalent character in ionic compounds is governed by Fazanís Rule.
(i) larger the charge on the ions.
(ii) smaller the size of anions.
(iii) larger the size of anion.
(iv) larger the polarizing power larger the covalent character.
AlCl3 will show Maximum covalent character on account of the higher polarising power of Al3+ because of its having higher positive charge and smaller size.