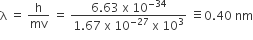In a fuel cell methanol is used as fuel and oxygen gas is used as an oxidizer. The reaction is
CH3OH(l) + 3/2O2(g) → CO2(g) + 2H2O(l)
At 298 K standard Gibb’s energies of formation for CH3OH(l), H2O(l) and CO2(g) are –166.2, –237.2 and –394.4 kJ mol–1 respectively. If
standard enthalpy of combustion of methanol is –726 kJ mol–1, efficiency of the fuel cell will be:
-
80%
-
97%
-
87%
-
90%
B.
97%
CH3OH(l) + 3/2O2(g) → CO2(g) + 2H2O(l)
Also ∆G°f CH3 OH( l) = -166.2 kJ mol-1
∆Gf°H2O (l ) = -237.2 kJ mol-1
∆Gf°CO2 (l ) = -394.4 kJ mol-1
∆G = Σ∆Gf° products −Σ∆Gf° reactants
= -394.4 -2 (237.2) + 166.2
= −702.6 kJ mol-1
now Efficiency of fuel cell = ∆G/∆H×100
= (702.6/726) x100
= 97%