The pKa of a weak acid (HA) is 4.5. The pOH of an aqueous buffered solution of HA in which 50% of the acid is ionized is
-
4.5
-
2.5
-
9.5
-
7.0
C.
9.5
For buffer solution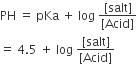
as HA is 50% ionized so [Salt] = [Acid]
pH = 4.5 pH + pOH = 14
⇒ pOH = 14 – 4.5 = 9.5






