How many moles of magnesium phosphate, Mg3(PO4)2 will contain 0.25 mole of oxygen atoms?
-
0.02
-
3.125 × 10–2
-
1.25 × 10–2
-
2.5 × 10–2
B.
3.125 × 10–2
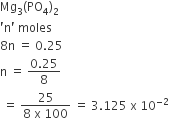
Sponsor Area
How many moles of magnesium phosphate, Mg3(PO4)2 will contain 0.25 mole of oxygen atoms?
0.02
3.125 × 10–2
1.25 × 10–2
2.5 × 10–2
B.
3.125 × 10–2
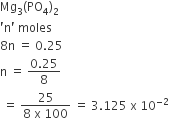
Sponsor Area
According to Bohr’s theory, the angular momentum of an electron in 5th orbit is
25h/ π
1.0h/π
10/π
2.5h/π
D.
2.5h/π
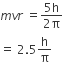
Which of the following molecules/ions does not contain unpaired electrons?

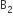
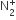

A.

Uncertainty in the position of an electron (mass = 9.1 × 10–31 kg) moving with a velocity 300 ms–1, accurate upto 0.001%, will be
19.2 × 10–2 m
5.76 × 10–2 m
1.92 × 10–2 m
3.84 × 10–2 m
C.
1.92 × 10–2 m
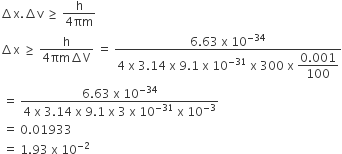
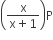
Phosphorus pentachloride dissociates as follows, in a closed reaction vessel,
PCl5 (g) ⇌ PCl3(g) + cl2(g)
If total pressure at equilibrium of the reaction mixture is P and degree of dissociation of PCl5 is x, the partial pressure of PCl3 will be
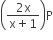

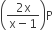
A.

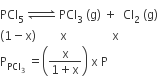
Sponsor Area
Mock Test Series