JEE Chemistry
Sponsor Area
Among the following, the maximum covalent character is shown by the compound
-
FeCl2
-
SnCl2
-
AlCl3
-
MgCl2
C.
AlCl3
The covalent character in ionic compounds is governed by Fazanís Rule.
(i) larger the charge on the ions.
(ii) smaller the size of anions.
(iii) larger the size of anion.
(iv) larger the polarizing power larger the covalent character.
AlCl3 will show Maximum covalent character on account of the higher polarising power of Al3+ because of its having higher positive charge and smaller size.
Sponsor Area
The hybridization of orbitals of N atom in NO3-, NO2+ and NH4+ are respectively
-
sp, sp2,sp3
-
sp2 , sp, sp
-
sp, sp3 , sp2
-
Sp2, sp3, sp
B.
sp2 , sp, sp
NO2+
Number of electron pairs = 2
Number of bond pairs = 2
Number of lone pair = 0
So, the species is linear with sp hybridisation.
NO3-
Number of electron pairs = 3
Number of bond pairs = 3
Number of lone pair = 0
So, the species is trigonal planar with sp2 hybridisation
NH4+
Number of electron pairs = 4
Number of bond pairs = 4
Number of lone pair = 0
So, the species is tetrahedral with sp3 hybridisation.
A gas absorbs a photon of 355 nm and emits at two wavelengths. If one of the emission is at 680 nm, the other is at
-
1035 nm
-
325 nm
-
743 nm
-
518 nm
C.
743 nm
E = E1 + E2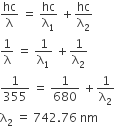
The entropy change involved in the isothermal reversible expansion of 2 moles of an ideal gas from a volume of 10 dm3 to a volume of 100 dm3 at 27°C is
-
38.3 J mol-1 K-1
-
35.8 J mol-1 K-1
-
32.3 J mol-1 K-1
-
42.3 J mol-1 K-1
A.
38.3 J mol-1 K-1

'a' and 'b' are van der Waals constants for gases. Chlorine is more easily liquefied than ethane because :
-
a and b for Cl2 > a and b for C2H6
-
a and b for Cl2 < a and b for C2H6
-
a and Cl2 < a for C2H6 but b for Cl2 > b for C2H6
-
a for Cl2 > a for C2H6 but b for Cl2 < b for C2H6
D.
a for Cl2 > a for C2H6 but b for Cl2 < b for C2H6
Vander Waals, constant a is due to force of attraction and b due to the infinite size of molecules. Thus, greater the value a and smaller the value b, larger the liquefaction.
| a | b | |
| Cl2 | 6.579 L2 bar mol-2 | 0.05622 L bar mol-2 |
| C2H5 | 5.562 L2 bar mol-2 | 0.06380 L mol-1 |
Sponsor Area
Mock Test Series
Mock Test Series





