ICSE physics
Sponsor Area
i) Give an example of a non-contact force which is always attractive in nature.
ii) How does the magnitude of this non-contact force n the two bodies depend on the distance of separation between them?
i) Gravitational force is attractive in nature.
ii) The magnitude of non-contact forces acting on two bodies depend on the distance of separation between them.
Force is inversely proportional to the distance of separation.
That is, the magnitude of force decreases with an increase in separation and force increases as the separation decreases.
Sponsor Area
A boy weighing 40 kgf climbs up a stair of 30 steps each 20 cm high in 4 minutes and a girl weighing 30 kgf does the same in 3 minutes.
Compare:
(i) The work done by them.
(ii) The power developed by them
Given:
Weight of the boy = force of gravity of the boy = 40 kgf
Time taken by the boy,tb = 4minutes = 4 x 60 s = 240 s
Weight of the girl= Force of gravity of the girl, Fg = 30 kgf
Time taken by the girl, tg = 3 minutes = 3 x 60 s = 180 s
Distance covered by both in 30 steps is,
D = 30 x 20 = 600 cm
While climbing upstairs, both have to do work against the force of gravity.
i) Work done by the boy in climbing the stairs is,
Wb = F x D = 40 kgf x 600 cm
i.e., Wb = 24000 J
Work done by the girl in climbing the stairs is:
Wg = F x D = 30 kgf x 600 cm
Wg = 18000 J
Therefore,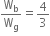
ii) Power developed = 
Boy:
Power developed = 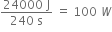
Girl:
Power developed = 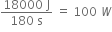
Therefore, the power developed by them is 1:1
With reference to the terms Mechanical Advantage, Velocity Ratio and efficiency of a machine, name and define the term that will not change for machine of a given design.
Velocity ratio will not change for the machine of a given design.
It is defined as the ratio of the velocity of effort to the velocity of the load.
Mathematically,
V.R. = 
Calculate the mass of ice required to lower the temperature of 300 g of water from 40o C to water at 0o C.
[Specific latent heat of ice = 336 J/g, specific heat capacity of water = 4.2 J/go C]
Let m be the mass of the ice that has to be added.
Heat energy required to melt to lower the temperature = mL = m x 336
Heat energy imparted by the water in fall of its temperature from 40o C to 0o C
= mass of water x specific heat capacity x fall in temperature
= 300 x 4.2 x 40o
If there is no loss of heat,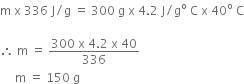
What do you understand by the following statements:
i) The heat capacity of the body is 60 JK-1
ii) The specific heat capacity of lead is 130 Jkg-1 K-1
i) Heat capacity is the amount of heat required to raise the temperature of a body by 1o C or 1 K.
Thus, by this statement, we mean that the amount of heat that is required to raise the temperature of a body by 1 K is 60 JK-1.
ii) Specific heat capacity is the amount of heat energy required to raise the temperature of a unit mass of a substance through 1o C or 1 K. Thus, 130 J kg-1 K-1 is the amount of heat energy required to raise the temperature of unit mass of lead through 1 K.
Sponsor Area
Mock Test Series
Mock Test Series





