ICSE chemistry
Sponsor Area
Choose the most appropriate answer from the following option:
Among the period 2 elements, the element which has high electron affinity.
-
Lithium
-
Carbon
-
Chlorine
-
Fluorine
D.
Fluorine
Sponsor Area
|
Group Number |
IA |
IIA |
IIIA |
IVA |
VA |
VIA |
VIIA |
0 18 |
|
2nd Period |
Li |
|
D |
|
|
O |
J |
Ne |
|
|
A |
Mg |
E |
Si |
|
H |
M |
|
|
|
R |
T |
I |
|
Q |
u |
|
y |
* In this table H does not represent Hydrogen.
* Some elements are given in their own symbol and position in the periodic table.
* While others are shown with a letter.
With reference to the table answer the following question:
I) Identify the most electronegative element.
II) Identify the most reactive elements of group 1.
III) Identify the element from period 3 with least atomic size.
IV) How many valence electrons are present in Q?
V) Which element from group 2 would have the least ionisation energy?
VI) Identify the noble gas of the fourth period.
VII) In the compound of A and H, what type of bond would be formed and give the molecular formula for the same?
I) J is a most electronegative element.
II) R is a most reactive element in group 1.
III) M has a least atomic size in period 3.
IV) 5 valence electrons are persent in Q.
V) T have least ionisation energy in group 2.
VI) y is noble gas in the fourth period.
VII) Ionic Bond.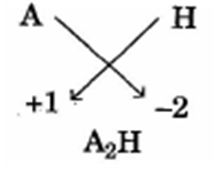
Identify the following substance which is underlined:
The element which has the highest ionisation potential.
Helium (He) has highest ionisation potential.
Give suitable chemical terms for the following;
A bond formed by a shared pair of electrons with both electrons coming from the same atom.
Coordinate bond is formed.
Choose the most appropriate answer from the following option:
Among the following compounds identify the compound that has all three bonds (ionic, covalent and coordinate bond).
-
Ammonia
-
Ammonium chloride
-
Sodium hydroxide
-
Calcium chloride
B.
Ammonium chloride
Sponsor Area
Mock Test Series
Mock Test Series





