ICSE chemistry
Sponsor Area
Select the correct answer from the choices A, B, C and D which is given.
Write only the letter corresponding to the correct answer .
Among the period 2 element the one which has high electron affinity is:
-
Lithium
-
Carbon
-
Fluorine
-
Neon
C.
Fluorine
Sponsor Area
Consider the selection of the periodic table given below:
|
Group Number |
IA 1 |
IIA 2 |
IIIA 13 |
IVA 14 |
VA 15 |
VIA 16 |
VIIA 17 |
0 18 |
|
2nd Period |
Li |
|
D |
|
|
O |
J |
Ne |
|
|
A |
Mg |
E |
Si |
|
H |
K |
|
|
|
B |
C |
|
F |
G |
|
|
L |
Note: IN this table B does not represent boron;
C does not represent Carbon
F does not represent fluorine
H does not represent Hydrogen
K does not represent potassium.
You must see the position of the element in the periodic table.
Some elements are given in their own symbol and position in the periodic table, while others are shown with a letter. With reference to the table;
i) Which is the most electronegative?
ii) How many valence electrons are present G?
iii) Write the formula of the compound between B and H.
iv) In the compound formed between F and J, what type of bond will be formed? Covalent
v) Draw the electron dot structure for the compound formed between C and K : diagram
i) J is the most electronegative.
ii) Five valence electron are present in G.
iii) B2H
iv) If the compound formed between F and J then the covalent bond is formed.
v) 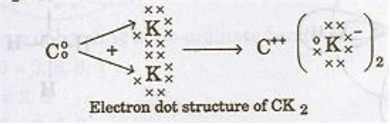
Define Ionization potential.
Ionisation potential : It is the amount of energy required to remove a valence electron from an isolated gaseous atom of an element.
Select the correct answer from the choice A, B,C and D which are given:
Among the following the one which is composed of all the three kinds of bond (ionic, covalent and coordinate bond)is:
-
Sodium chloride
-
Ammonia
-
Carbon tetrachloride
-
Ammonium chloride
A.
Sodium chloride
D.
Ammonium chloride
Methane is the first member of alkane when it is treated with an excess of chlorine in the presence of diffused sunlight forms carbon tetrachloride. Draw the appropriate structural formula of carbon tetrachloride and state the type of bond present in it.
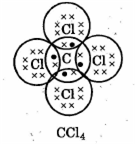
In carbon tetrachloride structure, covalent bond is present.
Sponsor Area
Mock Test Series
Mock Test Series





