CBSE chemistry
Sponsor Area
What is the effect of temperature on chemisorption?
Chemisorption increases with increase in temperature at a certain level and then starts decreasing.
Sponsor Area
What is the role of zinc metal in the extraction of silver?
In the extraction of silver, zinc metal act as reducing agent.
2[Ag(CN)2]-(aq.) + Zn(s) --> 2Ag(s) + [Zn(CN)4]2-(aq.)
What is the basicity of H3PO3?
H3PO3 ionises to give two H+ ions. Thus, it is dibasic in nature.
H3PO3 --> 2H+ + HPO2-3
Identify the chiral molecule in the following pair:

A chiral molecule in which one of the carbon atom bearing four different groups. In the following pair of molecules, the chiral molecule is

An element with density 2.8 g cm−3 forms of the f.c.c. unit cell with edge length 4 X10−8 cm. Calculate the molar mass of the element.
(Given: NA = 6.022 X 1023 mol −1)
Edge length, a = 4 x 10-8 cm
Density, d = 2.8 g cm-3
As the lattice is fcc type, the number of atoms per unit cell, z is 4.
Avogadro's number, NA = 6.022 x 1023 mol-1
Molar mass can be calculated with the help of given relation:
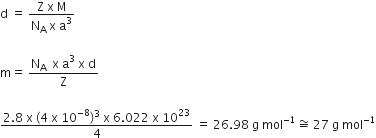
Sponsor Area
Mock Test Series
Mock Test Series





