CBSE chemistry
Sponsor Area
What are n-types semiconductors?
The semiconductor whose increased conductivity is a result of negatively-charged electrons is called an n-type semiconductor. These are generated when the crystal of a group 14 element such as Si or Ge is doped with a group 15 element such as P or As.
Sponsor Area
Define ‘peptization’.
Peptization can be defined as a process of converting a precipitate into colloidal sol by shaking it with dispersion medium in the presence of small amount of electrolyte.The electrolyte used in this process is known as a peptizing agent.
How is copper extracted from a low-grade ore of it?
Copper can be obtained from low grade from low through the process of leaching using acid or bacteria (leaching processes in which ore is treated with a suitable reagent which dissolves ore but not the impurities).
What is the basicity of H3PO2 acid and why?
Basicity of H3PO2 depends on upon the number of ionizable -OH groups present in the molecule. That is the number of hydrogen attached to the electronegative atom oxygen.
H3PO2 has one ionizable –OH group, thus its basicity is 1.
The structure of H3PO2 is as follows:

A reaction is of second order with respect to a reactant. How is its rate affected if the concentration of the reactant is (i) doubled (ii) reduced to half?
Let the concentration of the reactant be [A] = a
Rate of reaction, R = k [A]2 = ka2
(i) If the concentration of the reactant is doubled, i.e. [A] = 2a, then the rate of the reaction would be
R = k (2a)2
= 4ka
= 4R
Therefore, the rate of the reaction would increase by 4 times.
(ii) If the concentration of the reactant is reduced to half, i.e., ![]() then the rate of the reaction would be
then the rate of the reaction would be
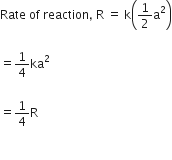
Therefore, the rate of the reaction would be reduced to ![]()
Sponsor Area
Mock Test Series
Mock Test Series





