CBSE science
Sponsor Area
Draw the structure for ethanoic acid molecules,CH3COOH.
Ethanoic acid [CH3COOH]
Sponsor Area
How does the valency of elements vary (i) in going down a group, and (ii) in going from left to right in a period of the periodic table ?
i) The valency remains same in going down the group.
ii) The valency first increases from 1to 4 and then decrease 4 to 0, in going from left to right in the period of the periodic table.
In the modern periodic table, the element calcium (atomic number = 20) is surrounded by elements with atomic number 12,19,21 and 38. Which of these elements has physical and chemical properties resembling those of calcium and why?
The electronic configuration of calcium (=20) 2,8,8,2.
The electronic configuration of other elements
atomic number 12 = 2,8,2
atomic number 19 = 2,8,8,1
atomic number 21 =2,8,8,3
atomic number 38= 2,8,18,8,2
The element having atomic number 12 and 38 resemble physical and chemical properties with calcium (20). This is because the valence electron of in these element is same as calcium (.e.=2) and the physical and chemical properties depend on the number of valence electron present in the outermost shell.
Write chemical equations for what happen when
i) sodium metal is added to ethanoic acid.
ii) sodium carbonate is added to ethanoic acid
iii) ethanoic acid reacts with a dilute solution of sodium hydroxide.
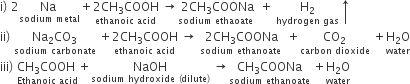
The atomic number of an element is 16.Predict
i) the number of valence electrons in its atom
ii)its valency
iii) its group number
iv) whether it is a metal or a non-metal
v) the nature of oxide formed by it
vi)the formula of its chloride
The electronic configuration of element 16: 2,8,6
i) The number of valence electrons 6.
ii)Its valency is 8-6 =2.
iii) Its group number is 16.
iv)It is a non-metal.
v) the nature of oxide is acidic.
vi) The valency of chloride is 1 and valency of X (=16) is 2 thus the formula is XCl2.
Sponsor Area
Mock Test Series
Mock Test Series





