Dual Nature Of Radiation And Matter
Sponsor Area
A 5 W source emits monochromatic light of wavelength 5000 A. When placed 0.5 m away, it liberates photoelectrons from a photosensitive metallic surface. when the source si moved to a distance of 1.0 m, the number of photoelectrons liberated will be reduced by a factor of:
-
4
-
8
-
16
-
2
A.
4
The intensity of light is inversely proportional to the square of the distance.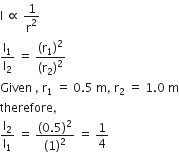
Now, since number of photoelectric emitted per second is directly proportional intensity, so number of electrons emitted would decrease by factor of 4.
Sponsor Area
A black body is at a temperature of 5760 K. The energy of radiation emitted by the body at wavelength 250 nm is U1, at wavelength 500 nm is U2 and that at 1000 nm is U3. Wien's constant, b = 2.88 x 106 nmK. Which of the following is correct?
-
U3 = 0
-
U1 > U2
-
U2 > U1
-
U1 = 0
C.
U2 > U1
Given, temperature, T1 = 5760 K
Given that energy of radiation emitted by the body at wavelength 250 nm in U1, at wavelength 500 nm is U2 and that at 1000 nm is U3.
Now, according to Wein's law, we get
where, b = Wien's constant = 2.88 x 106 nmK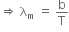

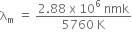

 is the wavelength corresponding to maximum energy, so U2 > U1.
is the wavelength corresponding to maximum energy, so U2 > U1.
A particle of mass 1 mg has the same wavelength as an electron moving with a velocity of 3 x 106 ms-1. The velocity of the particle is.
(mass of electron = 9.1 x 10-31 kg)
-
2.7 x 10-18 ms-1
-
9 x 10-2 ms-1
-
3 x 10-31 ms-1
-
2.7 x 10-21 ms-1
A.
2.7 x 10-18 ms-1
wavelength of a particle is given by
A photo-cell employs photoelectric effect to convert
-
change in the frequency of light into a change in electric voltage
-
change in the intensity of illumination into a change in photoelectric current
-
change in the intensity of illumination into a change in the work function of the photocathode
-
change in the frequency of light into a change in the electric current
B.
change in the intensity of illumination into a change in photoelectric current
In photoelectric effect when monochromatic radiations of suitable frequency fall on the photo-sensitive plate called cathode, the photoelectrons are emitted which get accelerated towards anode. These electrons flow in the outer circuit resulting in the photoelectric current.
Using the incident radiations of a fixed frequency, it is found that the photoelectric current increases linearly with the intensity of incident light as shown in figure. Hence, a photocell employs photoelectric effect to convert change in the intensity of illumination into a change in photoelectric current.
A photoelectric surface is illuminated successively by monochromatic light of wavelength λ and λ/2. If the maximum kinetic energy of the emitted photoelectrons in the second case is 3 times that in the first case, the work function of the surface of the material is
(h = planck's constant, c= speed of light)
-
hc/ 2λ
-
hc/λ
-
2 hc/λ
-
hc/3λ
A.
hc/ 2λ
According to Einstein photoelectric equation,
E = Kmax + Φ
Where Kmax is the maximum kinetic energy of emitted electron and Φ is work function of electrons.
Kmax = E - Φ = hv - Φ
Kmax = 
Similarly, in the second case, maximum kinetic energy of emitted electron is 3 times that in the first case, we get
3Kmax 
solving EQs (i) and (ii), we get work function of an emitted electron from a metal surface.
Φ = hc/2λ
Sponsor Area
Mock Test Series
Mock Test Series





