Surface Chemistry
Sponsor Area
A plot of log x/m versus log p for the adsorption of a gas on a solid gives a straight line with slope equal to:
-
-log k
-
n
-
1/n
-
log k
C.
1/n
The empirical relation x/m = kp1/n put forward by Freundlich is known as Freundlich adsorption isotherm. Taking logarithm
![]()
If the following curve is plotted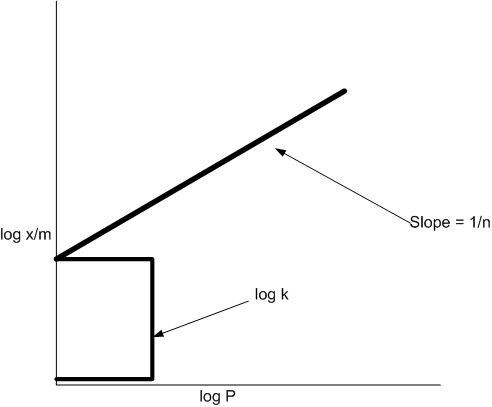
Sponsor Area
Fog is a colloidal solution of
-
Gas in liquid
-
Solid in gas
-
Gas in gas
-
Liquid in gas
D.
Liquid in gas
Fog is a colloidal solution of liquid in a gas in which liquid is the dispersed phase whereas gas is the dispersion medium.
The ease of adsorption of the hydrated alkali metal ions on an ion exchange resins follows the order
-
Li+ > K+ >Na+> Rb+
-
Rb+ < K+ < Na+ < Li+
-
K+ >Na+> Rb+ > Li+
-
Na+ < Li+ < K+ < Rb+
B.
Rb+ < K+ < Na+ < Li+
Ease of adsorption of the hydrated alkali metal ions on an ion-exchange resin decreases as the size of alkali metal ion increases.
since, the order of the size of alkali metal ions
Li+ < Na+ < K+ < Rb+
Thus, the ease of adsorption follows the order
Rb+ < K+ < Na+ < Li+
The Langmuir adsorption isotherm is deduced using the assumption:
-
the adsorption takes place in multilayers
-
The adsorption sites are equivalent in their ability to adsorb the particles
-
The adsorbed molecules interact with each other
-
The adsorbed molecules interact with each other
A.
the adsorption takes place in multilayers
The main points of Langmuir theroy of adsorption are as:
i) Adsorption takes place on the surface of the solid only till the whole of the surface is completely covered with a unimolecular layer of the adsorbed gas.
ii) Adsorption consist fo two opposing processes (a) condensation, and (b) evaporation.
iii) The rate of condensation depend upon the uncovered surface of the adsorbent available for condensation.
The protecting power of lyophilic colloidal sol is expressed in terms of
-
Coagulation value
-
Gold number
-
Critical micelle concentration
-
Oxidation number
B.
Gold number
Lyophobic sols are unstable, so they re-stabilised by adding some lyophilic colloids which protect them from precipitation. Thus, lyophilic colloids are called protecting colloids. Their protecting power is expressed in terms of gold number.
[Lesser the gold number, higher si the protecting power,]
Sponsor Area
Mock Test Series
Mock Test Series





