Redox Reactions
Sponsor Area
(I)H2O2 +O3 --> H2O +2O2
(II) H2O2 +Ag2O +--> 2Ag +H2O +O2
Role of hydrogen peroxide in the above reaction is respectively
-
oxidising in (I) and reducing (II)
-
reducing (I) and oxidizing in (II)
-
reducing in (I) and (II)
-
oxidising in (I) and (II)
A.
oxidising in (I) and reducing (II)
In the reaction,
since H2O2 oxidise, O3 into O2 thus it behaves as an oxidising agent.
the further reaction, in the reaction,
Here H2O2 reduces Ag2O into metallic silver [Ag] (as oxidation number is reducing from +1 to 0).Thus, H2O2 behaves as a reducing agent.
Sponsor Area
A mixture of potassium chlorate, oxalic acid and sulphuric acid is heated. During the reaction which element undergoes a maximum change in the oxidation number?
-
S
-
H
-
Cl
-
C
C.
Cl
When a mixture of potassium chlorate, oxalic acid and sulphuric acid is heated, the following reaction occurs:
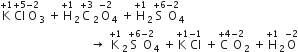
Thus, Cl is the element which undergoes a maximum change in the oxidation state.
In which of the following compounds, nitrogen exhibits highest oxidation state?
-
N2H4
-
NH3
-
N3H
-
NH2OH
C.
N3H
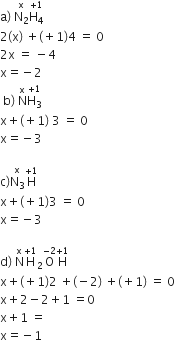
Thus, oxidation state of nitrogen is highest in N3H.
When Cl2 gas reacts with hot and concentrated sodium hydroxide solution, the oxidation number of chlorine changes from
-
zero to +1 and zero to -5
-
zero to -1 and zero to +5
-
zero to -1 and zero to +5
-
zero to +1 and zero to -3
B.
zero to -1 and zero to +5
When chlorine gas reacts with hot and concentrated NaOH solution, it disproportionates into Chloride (Cl-) and Chlorate (ClO3-) ions. 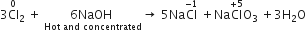
For the redox reaction
The correct coefficients of the reactants for the balanced equation are
H+ 16 5 2 MnO4- C2O42- H+ 2 5 16 MnO4- C2O42- H+ 5 16 2 MnO4- C2O42- H+ 2 16 5
B.
| MnO4- | C2O42- | H+ |
| 2 | 5 | 16 |
Sponsor Area
Mock Test Series
Mock Test Series





