Hydrogen
Sponsor Area
A hydrogen gas electrode is made by dipping platinum wire in a solution of HCl of pH =10 and by passing hydrogen gas through the platinum wire at 1 atm pressure. The oxidation potential of electrode would be
-
0.0591 V
-
0.59 V
-
0.118 V
-
1.18 V
B.
0.59 V
For Hydrogen electrode, oxidation half reaction is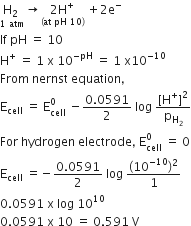
Sponsor Area
Some statement about heavy water are given below
A) Heavy water is used as a moderator in nuclear reactors.
B) Heavy water is more associated than ordinary water.
C) Heavy water is more effective solvent than ordinary
-
A and B
-
A, B and C
-
B and C
-
A and C
A.
A and B
Heavy water is used as a moderator in nuclear reactors. Its boiling point is higher as compared to the ordinary water. Thus, it is more associated as compared to ordinary water. The dielectric constant is however higher for H2O, thus, H2O is a more effective solvent than heavy water (D2O).
The reaction of aqueous KMnO4 with H2O2 in acidic conditions gives.
-
Mn4+ and O2
-
Mn2+ and O2
-
Mn2+ and O3
-
Mn4+ and MnO2
B.
Mn2+ and O2
The reaction of aqueous KMnO4 with H2O2 in acidic medium is
3H2SO4 + KMnO4 +5 H2O2 --> 5O2 +2MnSO4 +8H2O +K2SO4
In the reaction, KMnO4 oxidise H2O2 to O2 and itself [MnO4-] gets reduced to Mn2+ ions as MnSO4. Hence, aqueous solution of KMnO4 with H2O2 yields Mn2+ and O2 in acidic conditions.
Which of the following statement about hydrogen is incorrect?
-
Hydrogen never acts as cation in ionic salts
-
Hydronium ion, H3O+ exist freely in solution
-
Dihydrogen does not act as a reducing agent
-
Hydrogen has three isotopes of which tritium is the most common
C.
Dihydrogen does not act as a reducing agent
We can have both answers (C, D)
For ionic salts, hydrogen never behaves as cation, but behaves as anion (H-)
H3O+ exist freely in solution
Dihydrogen acts as a reducing agent.
Hydrogen has three isotopes. Protium, Deuterium and Tritium. Protium is the most common isotopes of hydrogen with an abundance of 99.98%.
Which one of the following molecular hydrides acts as a Lewis acid?
-
NH3
-
H2O
-
B2H6
-
CH4
C.
B2H6
Electron deficient molecules behave as Lewis acid.
Among the given molecules, only diborane is electron deficient, ie, does not have a complete octet. Thus, it acts as a Lewis acid.
NH3 and H2O being electron rich molecules behave as Lewis base.
Sponsor Area
Mock Test Series
Mock Test Series





