General Principles And Processes Of Isolation Of Elements
Sponsor Area
"Metals are usually not found as nitrates in their ores"
Out of the following two (a and b) reasons which isare/are true for the above observation?
I. Metal nitrates are highly unstable.
II.Metal nitrates are highly soluble in water.
-
I and II are true
-
I and II are false
-
I is false but II is true
-
I is true but II is false
C.
I is false but II is true
Metals are usually not found as nitrates in their ores, because metal nitrates are highly soluble in water. For example, KNO3 (salt peter) would be classified as completely soluble. Thus, KNO3 could be expected to dissociate completely in aqueous solution into K+ and NO3- ions.
![]()
The nitrate anion has three equivalent oxygen surrounding a central nitrogen atom. This tends to spread the single negative charge and make it easier for water to separate the ions in solution.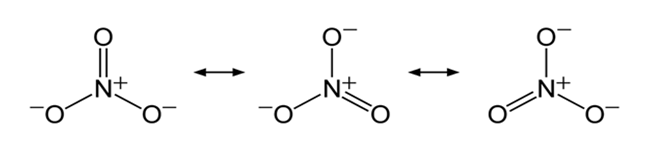
Sponsor Area
Al2O3 can be converted to anhydrous AlCl3 by heating:
-
Al2O3 with HCl gas
-
Al2O3 with NaCl in solid state
-
a mixture of Al2O3 and carbon in dry Cl2 gas
-
Al2O3 with Cl2 gas
C.
a mixture of Al2O3 and carbon in dry Cl2 gas
Al2O3 may be converted to anhydrous AlCl3 by heating of mixture of Al2O3 and carbon in dry chlorine
![]()
Aluminium is extracted from alumina (Al2O3) by electrolysis of a molten mixture of
-
Al2O3 +HF +NaAlF4
-
Al2O3 + CaF2 +NaAlF4
-
Al2O3 +Na3AlF6 +CaF2
-
Al2O3 +KF +Na3AlF6
C.
Al2O3 +Na3AlF6 +CaF2
Alumina, Al2O3, is a bad conductor of electricity and has a very high melting point, so before subjecting to electrolysis, it is mixed with fluorspar (CaF2) and cryolite (Na3AlF6), which lower its melting point and make it more conducting.
Copper sulphates dissolves in excess of KCN to give:
-
CuCN
-
[Cu(CN)4]3-
-
[Cu(CN)4]2-
-
Cu(CN)2
B.
[Cu(CN)4]3-
Copper sulphate on reaction with KCN to give cupric cyanide precipitates firstly which reduce into Cu2CN2 and dissolve in excess of KCN to give soluble K3[Cu(CN)4] complex salt
[CuSO4 + 2KCN →Cu(CN)2 + K2SO4] x 2
2Cu(CN2) → Cu2(CN)2 + NC - CN
Cu2(CN)2 + 6KCN → 2K3[Cu(CN)4]
_______________________________________
2CuSO4 + 10 KCN → 2K3[Cu(CN)4 + 2K2SO4 + (CN)2]
Extraction of gold and silver involves leaching with CN– ion. Silver is later recovered by
-
Liquation
-
Distillation
-
Zone refining
-
Displacement with Zn
D.
Displacement with Zn
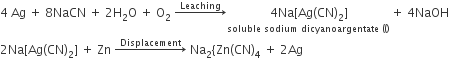
Sponsor Area
Mock Test Series
Mock Test Series





