The Solid State
Sponsor Area
A given metal crystallises out with a cubic structure having edge length of 361 pm. If there are four metal atoms in one unit cell, what is the radius of one atom.
-
40 pm
-
127 pm
-
80 pm
-
108 pm
B.
127 pm
Given, edge length = 361 pm
Four metal atoms in one unit cell
i.e effective number in unit cell (z) = 4 (given)
therefore,
It is a FCC structure
Face diagonal = 4r
![]()
Sponsor Area
A metal crystallises with a face-centered cubic lattice. The edge of the unit cell is 408 pm. The diameter of the metal atom is
-
288 pm
-
408 pm
-
144 pm
-
204 pm
A.
288 pm
For fcc lattice,
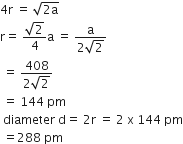
A metal has a fcc lattice. The edge length of the unit cell is 404 pm. The density of the metal is 2.72 g cm-3. The molar mass of the metal is (NA Avogadro's constant= 6.02 x 1023 mol-1)
-
40 g mol-1
-
30 g mol-1
-
27 g mol-1
-
20 g mol-1
C.
27 g mol-1
Given, cell is fcc, So Z =4
Edge length, a = 404 pm = 4.04 x 10-8 cm
Density of metal, d = 2.72 g cm-3
NA = 6.02 x 1023 mol-1
Molar mass ofg the metal, M =?
We know that
density, d=
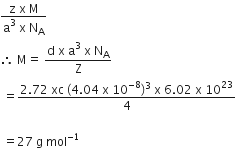
A solid compound XY has NaCl structure. If the radius of the cation is 100 pm, the radius of the anion (Y-) will be
-
275.1 pm
-
322.5 pm
-
241.5 pm
-
165.7 pm
C.
241.5 pm
A structure of a mixed oxide is cubic close packed (ccp). The cubic unit cell of mixed oxide is composed of oxide ions. One fourth of the tetrahedral voids are occupied by divalent metal A and the octahedral voids are occupied by a monovalent metal B. The formula of the oxide is
-
ABO2
-
A2BO2
-
A2B3O4
-
AB2O2
D.
AB2O2
According to ccp,
Number of O2- ions = 4
So, tetrahedral void = 8
and octahedral void = 4
Since A ions occupied 1/4th of the tetrahedral void.
Therefore,
Number of A ions = 1/4 x 8 = 2
Again, B ions occupied all octahedral void.
Therefore, Number of B ions = 4
A: B:O = 2:4:4
= 1:2:2
Structure of oxide= AB2O2
Sponsor Area
Mock Test Series
Mock Test Series





