Some Basic Concepts Of Chemistry
Sponsor Area
1.0 g of magnesium is burnt with 0.56 g of O2 in a closed vessel. Which reactant is left in excess and how much?
-
Mg, 0.16 g
-
O2, 0.16 g
-
Mg, 0.44 g
-
O2, 0.28 g
A.
Mg, 0.16 g
The balanced chemical equation is
Mg + 1/2O2 --> MgO
24g 16 g 40g
From the above equation, it is clear that,
24 f Mg reacts with 16 g O2
thus, 1.0 g Mg reacts with

Sponsor Area
10 g of hydrogen and 64 of oxygen were filled in a steel vessel and exploded. Amount of water produced in this reaction will be
-
2 mol
-
3 mol
-
4 mol
-
1 mol
C.
4 mol
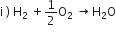
Amount of water produced is decided by limited reactant (ie, the reactant which is used in small amount)
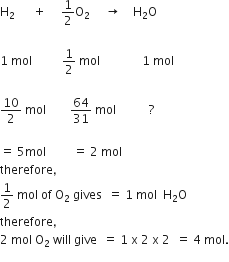
20.0 g of a magnesium carbonate sample decomposes on heating to give carbon dioxide and 8.0 g magnesium oxide. What will be the percentage purity of magnesium carbonate in the sample? (Atomic weight of Mg =24)
-
75
-
96
-
60
-
84
D.
84
In the given problem we have a practical yield of MgO. For calculation of percentage yield of MgO. we need a therortical yield of MgO. For this, we shall use mole concept.
MgCO3(s) → MgO (s) + CO2(g) .. (i)
6.02 x 1020 molecules of urea are present in 100 mL of its solution. The concentration of solution is
-
0.02 M
-
0.01 M
-
0.001 M
-
0.1 M
B.
0.01 M
Given, number of molecules of urea =6.02 x 1020
therefore ,Number of moles
= 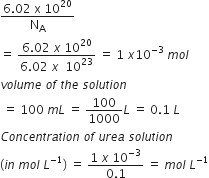
An element, X has the following isotopic composition;
200X: 90%
199X : 8.0%
202X ; 2.0 %
The weighted average atomic mass of the naturally -occurring element X is closet to:
-
200 amu
-
201 amu
-
202 amu
-
199 amu
A.
200 amu
Weight of 200X = 0.90 x 200 = 180.00 amu
Weight of 199X = 8.08 x 199 = 15.92 amu
Weight 0f 202X = 0.02 x 202 = 4.04 amu
Total weight = 199.06 = 200 amu
Sponsor Area
Mock Test Series
Mock Test Series





