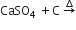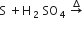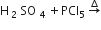Organic Chemistry – Some Basic Principles And Techniques
Sponsor Area
Considering the state of hybridization of carbon atoms, find out the molecule among the following which is linear.
-
CH3 - C ≡ C - CH3
-
CH2 = CH - CH2 - C ≡ CH
-
CH3 - CH2 - CH2 - CH3
-
CH3 - CH = CH - CH3
A.
CH3 - C ≡ C - CH3
CH3 - C ≡ C - CH3 linear, because C2 and C3 are sp hybridised.
Sponsor Area
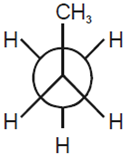
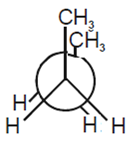
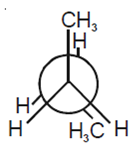
In the following the most stable conformation of n-butane is
B.
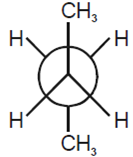
The conformation in which the heavier groups are present at maximum possible distances so that the forces of repulsion get weak is more stable.
Among the given conformations of n- butane, the conformation is shown in option (b) ie, anti conformation is most stable as in it the bulkier groups I(ie, CH3 group) are present at the maximum possible distance.
Liquid hydrocarbons can be converted to a mixture of gaseous hydrocarbons by
-
oxidation
-
cracking
-
distillation under reduced pressure
-
hydrolysis
B.
cracking
Lower hydrocarbon exists in a gaseous state while higher ones are in liquid state or solid state.
On cracking or pyrolysis, the hydrocarbon with higher molecular mass gives a mixture of hydrocarbons having lower molecular mass. Hence, we can say that by cracking a liquid hydrocarbon can be converted into a mixture of gaseous hydrocarbons.
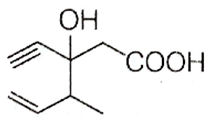
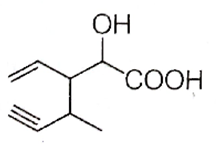
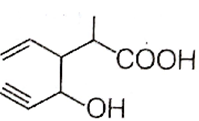
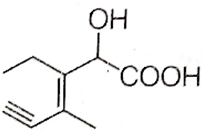
Structure of the compound whose IUPAC name is 3-ethyl-2-hydroxy-4-methylhex-3-en-5-ynoic acid is
B.
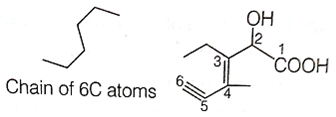
Sulphur trioxide can be obtained by which of the following reaction
B.
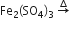
Sponsor Area
Mock Test Series
Mock Test Series