Coordination Compounds
Sponsor Area
[Co(NH3)4(NO2)2]Cl exhibits:
-
linkage isomerism, ionisation isomerism and optical isomerism
-
Linkage isomerism, ionisation isomerism and geometrical isomerism
-
ionization isomerism, geometrical isomerism and optical isomerism
-
linkage isomerism, geometrical isomerism and optical isomerism
B.
Linkage isomerism, ionisation isomerism and geometrical isomerism
The compound [Co(NH3)4((NO2)2]Cl exhibits linkage, ionisation and geometrical isomerism.
Hence, its linkage isomers are
(i) [Co(NH3)2(NO2)2]Cl and [Co(NH3)4(ONO)2]Cl
(ii) Its ionisation isomers are
[Co(NH3)4 (NO2)Cl]NO2 and [Co(NH3)4(NO2)2]Cl
(iii) Its geometrical isomers are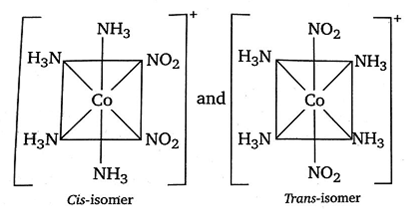
Sponsor Area
[Cr(H2O)6]Cl3 (at. no. of Cr = 24) has a magnetic moment of 3.83 BM, the correct distribution of 3d electrons in the chromium of the complex is:
C.
Magnetic moment,
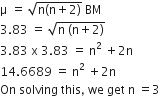
Hence, a number of unpaired electrons in d- subshell of the penultimate shell of chromium (Cr = 24).
so, the configuration of chromium ion is
Cr3+ = 1s2, 2s2, 2p6, 3s2, 3p6 3d3
In [Cr(H2O)6]Cl2 oxidation state of Cr is +3
Hence in 3d3 the distribution of electrons
![]()
A magnetic moment of 1.73 BM will be shown by one among the following
-
[Cu(NH3)4]2+
-
[Ni(CN)4]2-
-
TiCl4
-
[CoCl6]4-
A.
[Cu(NH3)4]2+

Among the following complexes, the one which shows zero crystal field stabilisation energy (CFSE) is
-
[Mn(H2O)6]3+
-
[Fe(H2O)6]3+
-
[Co(H2O)6]3+
-
[Co(H2O)]3+
B.
[Fe(H2O)6]3+
The CFSE for octahedral complex is given by
CFSE = [-0.4 t2g e- +0.6 ege-]
For Mn3+, [3d4]--> t2g3e1g
therefore,
CFSE = [-0.4 x 3 + 0.6 x1]
For Fe3+, [3d] --> t2g3e2g
CFSE = [(-0.4 x 3) + (0.6 x2)] = 0
For Co2+, [3d7] --> t2g5e2g
CFSE = [(-0.4 x 5) + (0.6 x2)] =-0.8
For Co3+, [3d6] ---> t2g4e2g
CFSE = [(-0.4 x4) + (0.6 x2)] = -0.4
An example of a sigma bonded organometallic compound is :
-
Ruthenocene
-
Grignard's reagent
-
Ferrocene
-
Cobaltocene
B.
Grignard's reagent
Grignard's reagent i.e., RMgX is σ-bonded organometallic compound.
Sponsor Area
Mock Test Series
Mock Test Series





