Amines
Sponsor Area
Among the following compounds, the one that is most reactive towards electrophilic nitration is
-
Benzoic acid
-
Nitrobenzene
-
toluene
-
benzene
C.
toluene
The presence of electron releasing group like -R, -OH etc., increases the electron density at o/p position and thus, makes the benzene ring more reactive (at(o/p position) towards electrophile. On the other hand, electron withdrawing group like-COOH,-NO2 etc. If present, reduces electron density and thus, reduces the activity benzene nucleus towards electrophile.Thus, the order of the given compounds towards electrophilic nitration is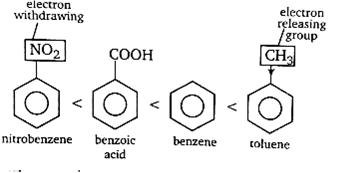
Thus, toluene is most reactive towards electrophilic nitration.
Sponsor Area
An organic compound (C3H9N) (A), when treated with nitrous acid, gave an alcohol and N2 gas was evolved. (A) on warming with CHCl3 and Caustic potash gave (C) which on reduction gave iso-propyl methylamine predict the structure of (A)
-

-
CH3-CH2 -NH -CH3
-
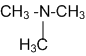
-
CH3CH2CH2-NH2
A.
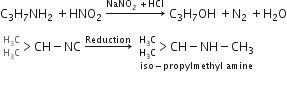
'A' is Isopropyl methylamine
Consider the nitration of benzene using mixed conc. H2SO4 and HNO3. If a large amount of KHSO4 is added to the mixture, the rate of nitration will be
-
slower
-
uncharge
-
doubled
-
faster
A.
slower
IN the nitration of benzene in the presence of conc. H2SO4 and HNO3, benzene is formed.
HNO3 +H2SO4 ![]()
If a large amount of KHSO4 is added to this mixture more HSO4- ion furnishes and hence the concentration of electrophile decreases, rate of electrophilic aromatic reaction slows down.
Consider the reaction,
RCHO +NH2NH2 →RCH =N-NH2
What sort of reaction is it?
-
Electrophilic addition elimination reaction
-
Free radical addition-elimination reaction
-
Electrophilic substitution elimination reaction
-
Nucleophilic addition-elimination reaction
D.
Nucleophilic addition-elimination reaction
In the above reaction
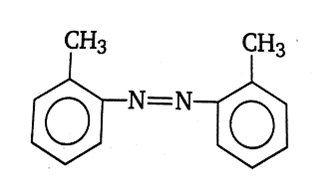
In a reaction of aniline, a coloured product C was obtained.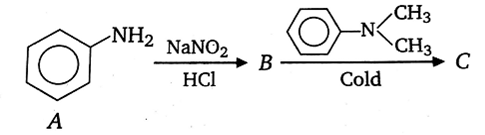
D.
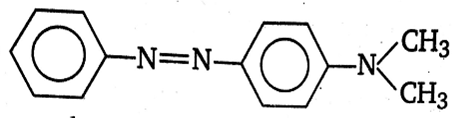
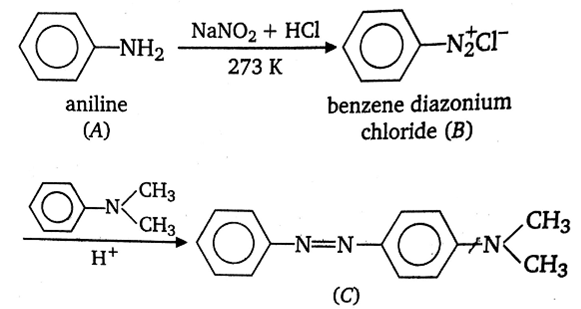
Sponsor Area
Mock Test Series
Mock Test Series