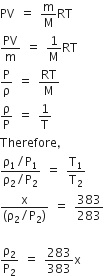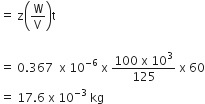A particle of mass M starting from rest undergoes uniform acceleration. If the speed acquired in time T is v, the power delivered to the particle is
-
Mv2/T
-
Mv2 / 2T2
-
Mv2/T2
-
Mv2 / 2T
D.
Mv2 / 2T
The kinetic energy of particle = Mv2 / 2
Power = Energy /Time
P = Mv2 /2T