A 5 W source emits monochromatic light of wavelength 5000 A. When placed 0.5 m away, it liberates photoelectrons from a photosensitive metallic surface. when the source si moved to a distance of 1.0 m, the number of photoelectrons liberated will be reduced by a factor of:
-
4
-
8
-
16
-
2
A.
4
The intensity of light is inversely proportional to the square of the distance.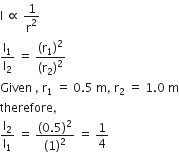
Now, since number of photoelectric emitted per second is directly proportional intensity, so number of electrons emitted would decrease by factor of 4.









 is the wavelength corresponding to maximum energy, so U2 > U1.
is the wavelength corresponding to maximum energy, so U2 > U1.

