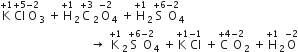(I)H2O2 +O3 --> H2O +2O2
(II) H2O2 +Ag2O +--> 2Ag +H2O +O2
Role of hydrogen peroxide in the above reaction is respectively
-
oxidising in (I) and reducing (II)
-
reducing (I) and oxidizing in (II)
-
reducing in (I) and (II)
-
oxidising in (I) and (II)
A.
oxidising in (I) and reducing (II)
In the reaction,
since H2O2 oxidise, O3 into O2 thus it behaves as an oxidising agent.
the further reaction, in the reaction,
Here H2O2 reduces Ag2O into metallic silver [Ag] (as oxidation number is reducing from +1 to 0).Thus, H2O2 behaves as a reducing agent.