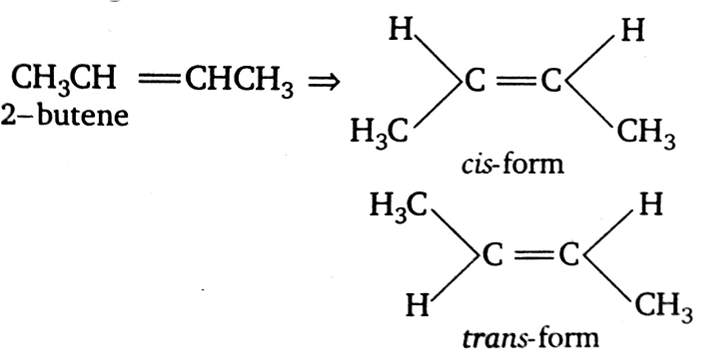
Which of the following compound will exhibit cis-trans (geometrical) isomerism?
-
2-butene
-
Butanol
-
2-butyne
-
2-butenol
A.
2-butene
Compounds which have at least one double bond (C=C) and the groups attached with double bonded carbon atoms are different exhibit geometrical isomerism.