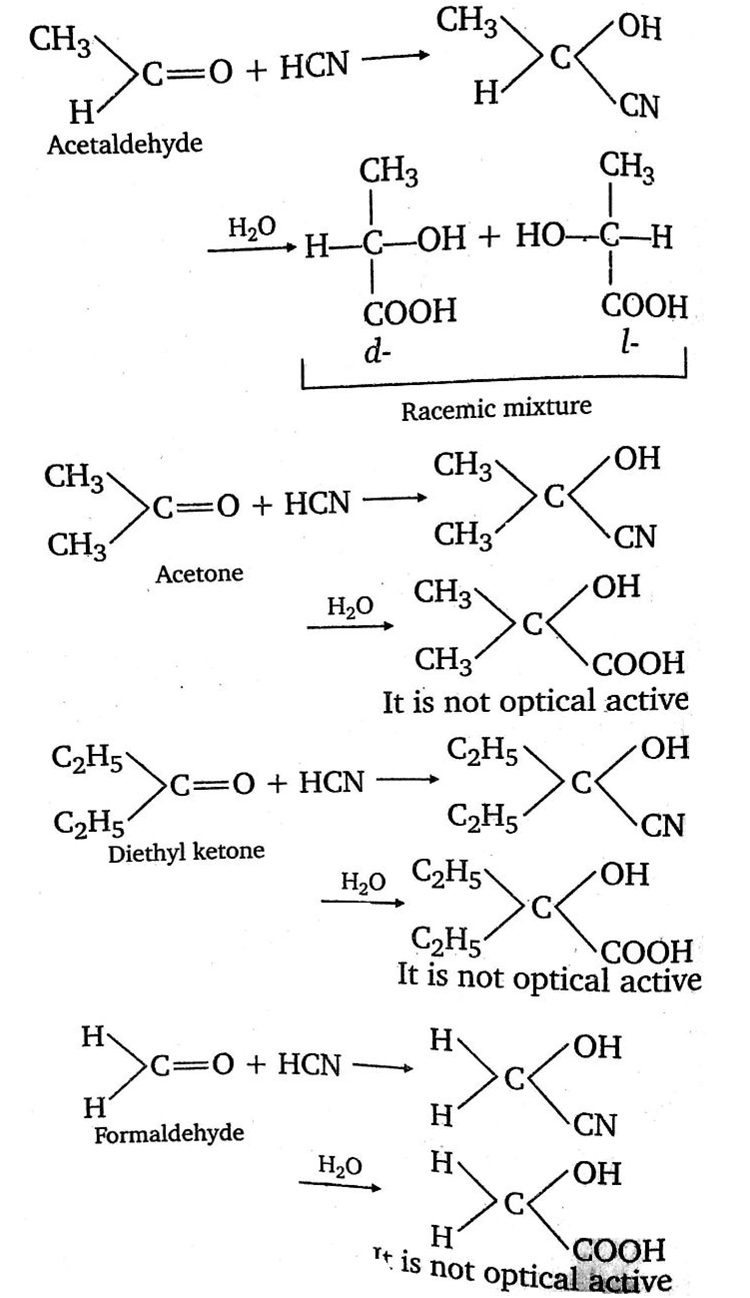
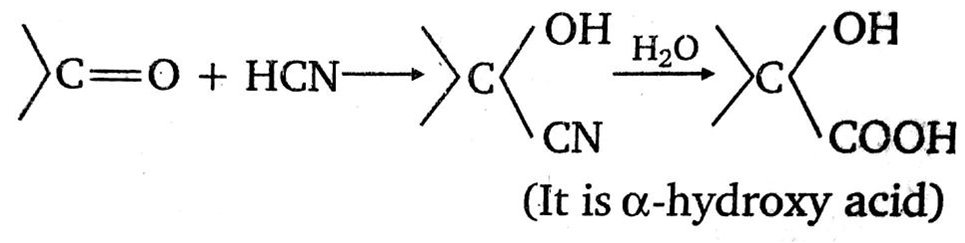A carbonyl compound reacts with hydrogen cyanide to form cyanohydrin which on hydrolysis forms a racemic mixture of alpha -hydroxy acid. The carbonyl compound is:
-
CH3-CH2-CH2COCH3
-
(CH3)2C=O
-
CH3CH2CHO
-
CH3CHO
D.
CH3CHO
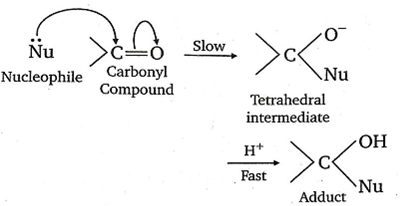
The carbonyl compounds undergo nucleophilic addition reaction because oxygen is more electronegative than carbon. As such, it withdraws share pi electron pair towards itself and gets a partial negative charge, therefore carbon gets a partial positive charge and becomes susceptible to nucleophilic attack.
The aldehyde is more reactive than ketones towards nucleophiles. This can be explained on the basis of inductive effect as well as steric effect. The addition of nucleophiles is based upon the positive charge present on a carbon atom of > C =O group.
In aldehyde >C=O group is present at least one alkyl group (except formaldehyde) which has +I effect (electron donating effect) and which decreases the positive charge of carbon, thereby making the attack to nucleophile difficult. The nucleophilic attack becomes more difficult in ketones having a minimum of two alkyl groups.
Hence, by means of attachment of alkyl groups (due to +I effect) rate of nucleophiles addition decreases.
Order of +I effect in an alkyl group.![]()
order of nucleophilic addition in given carbonyl compound is
CH3CHO > CH3-CH2- CHO > (CH3)2CO> CH3CH2CH2COCH3