Chemical Kinetics
Sponsor Area
A radioactive element gets spilled over the floor of a room. Its half-life period is 30 days. If the initial activity is ten times the permissible value, after how many days will it be safe to enter the room ?
-
1000 days
-
300 days
-
50 days
-
100 days
D.
100 days
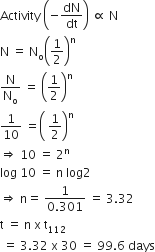
Sponsor Area
A reaction involving two different reactants can never be
-
Unimolecular reaction
-
First order reaction
-
second order reaction
-
Bimolecular reaction
A.
Unimolecular reaction
A reaction was found to be second order with respect to the concentration of carbon monoxide. If the concentration of carbon monoxide is doubled, with everything else kept the same, the rate of reaction will
-
remain unchanged
-
triple
-
increase by a factor of 4
-
double
C.
increase by a factor of 4
R∝ [W]2
R'∝ [2CO]2
R∝ 4[W]2
R∝ 4M
A schematic plot of In Keq versus inverse of temperature for a reaction is shown below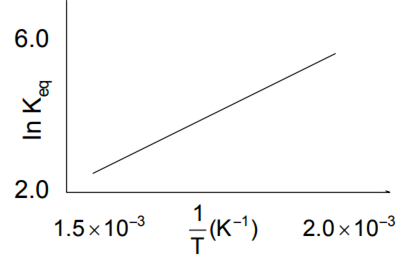
The reaction must be
-
exothermic
-
endothermic
-
one with negligible enthalpy change temperature
-
highly spontaneous at ordinary
A.
exothermic
An amount of solid NH4HS is placed in a flask already containing ammonia gas at a certain temperature and 0.50 atm. Pressure. Ammonium hydrogen sulphide decomposes to yield NH3 and H2S gases in the flask. When the decomposition reaction reaches equilibrium, the total pressure in the flask rises to 0.84 atm. The equilibrium constant for NH4HS decomposition at this temperature is
-
0.30
-
0.18
-
0.17
-
0.11
D.
0.11
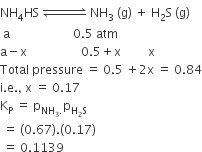
Sponsor Area
Mock Test Series
Mock Test Series





