Some Basic Concepts Of Chemistry
Sponsor Area
A gaseous hydrocarbon gives upon combustion 0.72 g of water and 3.08 g of CO2.The empirical formula of the hydrocarbon is
-
C2H4
-
C3H4
-
C6H5
-
C7H8
D.
C7H8
18 g H2O contains 2gH
therefore, 0.72 g H2O contains 0.08 g H
44 g CO2 contains 12 g C
therefore, 3.08 g CO2 contains 0.84 g C
∴ C:H = 0.84/12: 0.08/1
+ 0.07 : 0.08 = 7:8
∴Empirical formula = C7H8
Sponsor Area
Experimentally it was found that a metal oxide has formula M0.98O. Metal M, is present as M2+ and M3+ in its oxide. The fraction of the metal which exists as M3+ would be:
-
7.01%
-
4.08%
-
6.05%
-
5.08%
B.
4.08%
Metal oxide = M0.98O
If ‘x’ ions of M are in +3 state, then
3x + (0.98 – x) × 2 = 2
x = 0.04
So the percentage of metal in +3 state would be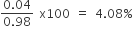
How many moles of magnesium phosphate, Mg3(PO4)2 will contain 0.25 mole of oxygen atoms?
-
0.02
-
3.125 × 10–2
-
1.25 × 10–2
-
2.5 × 10–2
B.
3.125 × 10–2
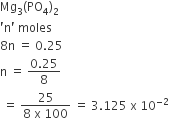
If we consider that 1/6 in place of 1/12 mass of carbon atom is taken to be the relative atomic mass unit, the mass of one mole of a substance will
-
Decrease twice
-
Increase two fold
-
Remain unchanged
-
Be a function of the molecular mass of the substance
C.
Remain unchanged
In Carius method of estimation of halogens, 250 mg of an organic compound gave 141 mg of AgBr. The percentage of bromine in the compound is: (at. Mass Ag = 108; Br = 80)
-
24
-
36
-
48
-
60
A.
24
Weight of Organic compound = 250 mg
Weight of AgBr = 141 mg
therefore, According to the formula of % of bromine by Carius method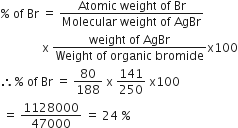
Sponsor Area
Mock Test Series
Mock Test Series





