Hydrocarbons
Sponsor Area
Question
At 300 K and 1 atm, 15 mL of a gaseous hydrocarbon requires 375 mL air containing 20% O2 by volume for complete combustion. After combustion the gases occupy 330 mL. Assuming that the water formed is in liquid form and the volumes were measured at the same temperature and pressure, the formula of the hydrocarbon is:
-
C3H8
-
C4H8
-
C4H12
-
C3H6
Solution
C.
C4H12
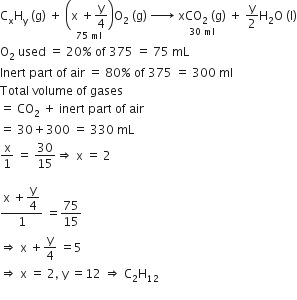
Sponsor Area
Sponsor Area
Mock Test Series
Mock Test Series





