Equilibrium
Sponsor Area
Among the following acids which has the lowest pKa value?
-
CH3COOH
-
HCOOH
-
(CH3)2COOH
-
CH3CH2COOH
B.
HCOOH
Sponsor Area
An ideal gas is allowed to expand both reversibly and irreversibly in an isolated system. If Ti is the initial temperature and Tf is the final temperature, which of the following statements is correct?
-
(Tf)irrev > (Tf)rev
-
Tf > Ti for reversible process but Tf = Ti for irreversible process
-
(Tf)rev = (Tf)irrev
-
Tf = Ti for both reversible and irreversible processes
A.
(Tf)irrev > (Tf)rev
At 25°C, the solubility product of Mg(OH)2 is 1.0 × 10–11. At which pH, will Mg2+ ions start precipitating in the form of Mg(OH)2 from a solution of 0.001 M Mg2+ ions?
-
9
-
10
-
11
-
8
B.
10
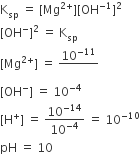
Consider the reaction:
Cl2(aq) + H2S(aq) → S (s) + 2H+ (aq) + 2Cl- (aq)
The rate equation for this reaction is
I. Cl2 + H2S → H+ +Cl- + Cl+ +HS-
II. H2S ⇌ H+ + HS- (fast equilibrium)
Cl2 + HS- → 2Cl- + H+ + S (slow)
-
II only
-
Both (I) and (II)
-
Neither (I) nor (II)
-
(I) only
D.
(I) only
For (A)
rate = K[Cl2] [H2S]
For (B)
rate = K[Cl2] [HS-] … (i)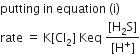
For a spontaneous reaction the ∆G, equilibrium constant (K) and Eocell will be respectively
-
-ve, >1, +ve
-
+ve, >1, -ve
-
-ve, <1, -ve
-
-ve, >1, -ve
A.
-ve, >1, +ve
Sponsor Area
Mock Test Series
Mock Test Series





