Coordination Compounds
Sponsor Area
A solution containing 2.675 of CoCl3. 6NH3(molar mass = 267.5 g mol–1) is passed through a cation exchanger. The chloride ions obtained is solution was treated with excess of AgNO3 to give 4.78 g of AgCl (molar mass = 143.5 g mol–1 ). The formula of the complex is
(At. mass of Ag = 108 u)
-
[Co(NH3)6]Cl3
-
[CoCl2(NH3)4]Cl
-
[CoCl3(NH3)3]
-
[CoCl(NH3)5]Cl2
A.
[Co(NH3)6]Cl3
CoCl. 6NH3 + AgNO3 → AgCl
2.675/2667.5 excess 4.78/143.5
= 0.01 mole = 0.03310 mole
because 0.01 mole CoCl3.6NH3 given 0.0331 mole AgCl.
hence 1 mole of CoCl3.6NH3 will given 0.03310/0.010 =3 mole.
Thus the formula of the compound will be [Co(NH3)6]Cl3
Sponsor Area
Among the properties (a) reducing (b) oxidising (c) complexing, the set of properties shown by CN- ion towards metal species is
-
a, b
-
a, b, c
-
c, a
-
b,c
C.
c, a
CN- is a better complexing agent (C) as well as a reducing agent (A)
Thus properties (A) and (C) are shown
Property (C) Ni2+ + 4 CN- → [Ni(CN)4]2-
Property (A) CuCl2 + 5 KCN → K3[Cu(CN)4] + 1/2 (CN)2 + 2KCl
(CN- reduces Cu2+ to Cu+ )
Amongst the following compound, the optically active alkane having lowest molecular mass
D.
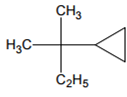
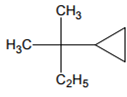
contains asymmetric carbon, thus optically active.
Coordination compound has great importance in biological systems. In this context which of the following statements is incorrect?
-
Chlorophylls are green pigments in plants and contain calcium
-
Carboxypeptidase – A is an enzyme and contains zinc
-
Cyanocobalamin is B12 and contains cobalt
-
Haemoglobin is the red pigment of blood and contains iron
A.
Chlorophylls are green pigments in plants and contain calcium
Chlorophyll contains Mg not Ca.
For which of the following parameters the structural isomers C2H5OH and CH3OCH3 would be expected to have the same values?
-
Heat of vaporization
-
Gaseous densities at the same temperature and pressure
-
Boiling points
-
Vapour pressure at the same temperature
B.
Gaseous densities at the same temperature and pressure
Sponsor Area
Mock Test Series
Mock Test Series