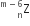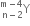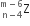Nuclei
Sponsor Area
A mixture consists of two radioactive materials A1 and A2 with half-lives of 20 s and 10 s respectively. Initially, the mixture has 40 g of A1 and 160 g of A2. The amount of the two in the mixture will become equal after
-
60 s
-
80 s
-
20 s
-
40 s
D.
40 s
For 40 g amount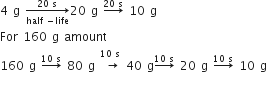
So, after 40 A1 and A2 remains same.
Sponsor Area
A nucleus 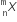 emits one α - particle and two β- particles. The resulting nucleus is
emits one α - particle and two β- particles. The resulting nucleus is
B.
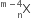
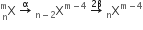
A nucleus has 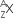 mass represented by M (A, Z). If Mp and Mn denote the mass of proton and neutron respectively and BE the binding energy (in MeV), then:
mass represented by M (A, Z). If Mp and Mn denote the mass of proton and neutron respectively and BE the binding energy (in MeV), then:
-
BE = [M(A,Z)-ZMp - (A-Z)Mn]c2
-
BE = [ZMp + (A-Z)Mn -M(A,Z)]c2
-
BE = [ZMp + AMn - M (A,Z)]c2
-
BE = M (A,Z)- ZMp - (A-Z) Mn
B.
BE = [ZMp + (A-Z)Mn -M(A,Z)]c2
In the case of formation of a nucleus, the evolution of energy equal to the binding energy of the nucleus takes place due to the disappearance of a fraction of the total mass. If the quantity of mass disappearing is ΔM, then the binding energy is
BE = ΔMc2
From the above discussion, it is clear that the mass of the nucleus must be less than the sum of the masses of the constituent neutrons and protons. We can then write.
ΔM= [ZMp + (A-Z)Mn -M(A,Z)
BE = [ZMp + (A-Z)Mn -M(A,Z)]c2
Where N = A -Z = number of neutrons.
A nucleus of uranium decays at rest into nuclei of thorium and helium. Then,
-
the helium nucleus has more kinetic energy than the thorium nucleus
-
the helium nucleus has less momentum than the thorium nucleus
-
the helium nucleus has more momentum than the thorium nucleus
-
the helium nucleus has less kinetic energy than the thorium nucleus
A.
the helium nucleus has more kinetic energy than the thorium nucleus

A radio isotope X with a half life 1.4 x 109 yr decays of Y which is stable. A sample of the rock from a cave was found to contain X and Y in the ratio 1:7. The age of the rock is,
-
1.96 x 109 yr
-
3.92 x 109 yr
-
4.20 x 109 yr
-
8.40 x 109 yr
C.
4.20 x 109 yr
Ratio of X:Y is given = 1:7
That is,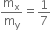
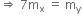
Let, the initial total mass is m.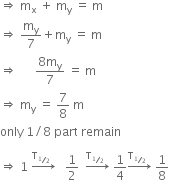
Therefore, time taken to become 1/8 unstable part
= 3 x T1/2
= 3 x 1.4 x 109
= 4.2 x 109 y
Sponsor Area
Mock Test Series
Mock Test Series