Thermodynamics
Sponsor Area
A gas is allowed to expand in a well-insulated container against a constant external pressure of 2.5 atm from an initial volume of 2.50 L to a final volume of 4.50 L. The change in internal energy ΔU of the gas in joules will be
-
1136.5 J
-
-500 J
-
-505 J
-
+505 J
C.
-505 J
ΔU = q + w
For adiabatic process, q = 0
∴ ΔU = w
= – P·ΔV
= –2.5 atm × (4.5 – 2.5) L
= –2.5 × 2 L-atm
= –5 × 101.3 J
= –506.5 J
= –505 J
Sponsor Area
A reaction having equal energies of activation for forward and reverse reactions has
- ΔS =0
- ΔG =0
- ΔH = 0
- ΔH = ΔG=ΔS = 0
C.
ΔH = 0Energy profile diagram for are reaction is as from the figure it is clear that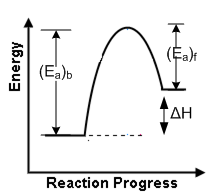
(Ea)b = (Ea)f +ΔH
[Here (Ea)b = activation energy of backward reaction and (Ea)f = activation energy of forward reaction].
If (Ea)b = (Ea)b = (Ea)f
then ΔH = 0
Assume each reaction is carried out in an open container. For which reaction will ΔH = ΔE?
-
H2 (g) + Br2 (g) →2HBr (g)
-
C (s) + 2 H2O (g) → 2 H2 (g) + CO2 (g)
-
PCl5 (g) →PCl3 (g) + Cl2 (g)
-
2CO (g) + O2 (g) → 2 CO2 (g)
A.
H2 (g) + Br2 (g) →2HBr (g)
As we know that
ΔH = ΔE + PΔV
ΔH = ΔE +ΔnRT ..(1)
where ΔH → change in enthalpy of the system (standard heat at constant pressure)
Δ E → change in internal energy of system (Standard heat at constant volume)
Δn → no. of gaseous moles of product - no. of gaseous moles of reactant
R → gas constant
T → absolute temperature
If Δ n = 0 for reactions which is carried out in an open container, therefore, Δn = 0 for reactions which are carried out in an open container, therefore, ΔH =ΔE
so for reaction (1) Δn = 2-2 = 0
Hence, for reaction (1) , ΔH =ΔE
Consider the following processes Δ H (kJ/mol)
1/2 A → +150
3B → 2 C + D -125
E + A → 2D +350
For B + D → E + 2C, ΔH will be
-
525 kJ/mol
-
-175 kJ/mol
-
-325 kJ /mol
-
325 kJ/mol
B.
-175 kJ/mol
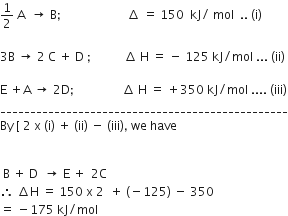
Consider the following reactions: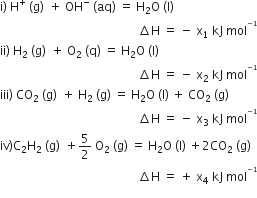
Enthalpy of formation of H2O (l) is:
-
- x2 kJ mol-1
-
+ x3 kJ mol-1
-
- x4 kJ mol-1
-
+ x1 kJ mol-1
A.
- x2 kJ mol-1
Enthalpy of formation: The amount of heat evolved or absorbed during the formation of 1 mole of a compound from its constituent elements is known as heat of formation. SO, the correct answer is: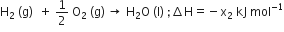
Sponsor Area
Mock Test Series
Mock Test Series





