States Of Matter
Sponsor Area
20-litre container at 400 K contains CO2(g) at pressure 0.4 atm and an excess of SrO (neglect the volume of solid SrO). The volume of the containers is now decreased by moving the movable piston fitted in the container. The maximum volume of the container, when pressure of CO2 attains its maximum value, will be
Given that: SrCO3 ⇌ SrO(s) + CO2(g) kp = 1.6 atm)
-
5 litre
-
10 litre
-
8 litre
-
3 litre
A.
5 litre
Max. the pressure of CO2= Pressure of CO2 at equilibrium
For reaction, SrCO3 ⇌ SrO(s) + CO2(g)
Kp= PCO2 = 1.6 atm = maximum pressure of CO2
Volume Container at this stage,
V= nRT/P .... (i)
Since container is sealed and reaction was not earlier at equilibrium
therefore, n = constant
n= Pv/RT .... (ii)
from equation (i) and (ii) we get
V = (0.4 x 20)/RT = 5 Litre
Sponsor Area
50 mL of each gas A and of gas B takes 150 and 200 s respectively for effusing through a pin hole under the similar conditions. If molecular mass of gas B is 36, the molecular mass of gas A will be
-
96
-
128
-
20.2
-
64
C.
20.2
Given,
VA =VB = 50 mL
TA = 150 s
TB = 200 s
MB = 36
MA = ?
From Graham's law of effusion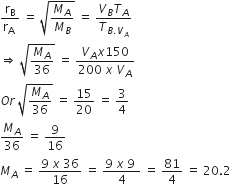
A certain gas takes three times as long to effuse out as helium. Its molecular mass will be
-
27 u
-
36 u
-
64 u
-
9 u
B.
36 u
From Graham's diffusion law,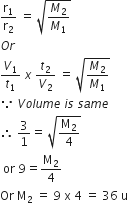
A bubble of air is underwater at temperature 15o C and the pressure 1.5 bar. If the bubbles rises to the surface where the temperature is 25o C and the pressure is 1.0 bar, what will happen to the volume of the bubble?
-
Volume will become greater by a factor of 1.6
-
Volume will become greater by a factor of 1.1
-
Volume will become smaller by a factor of 0.70
-
Volume will become greater by a factor of 2.9
A.
Volume will become greater by a factor of 1.6
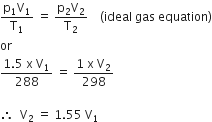
i.e., volume of bubble will be almost 1.6 times to initial volume of bubble.
A gas such as carbon monoxide would be most likely to obey the ideal gas law at
-
high temperatures and low pressures
-
low temperatures and high pressures
-
high temperatures and high pressures
-
low temperatures and low pressures
A.
high temperatures and low pressures
Real gases show ideal gas behaviour at high temperatures and low pressures.
PV = nRT
Sponsor Area
Mock Test Series
Mock Test Series





