Electrochemistry
Sponsor Area
A button cell used in watches functions as following
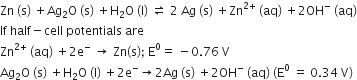
The cell potential will be
-
1.10 V
-
0.42 V
-
0.84 V
-
1.34 V
A.
1.10 V
Anode is always the site of oxidation thus anode half cell is
Zn2+ (aq) +2e- --> Zn (s); E0 =-0.76 V
Cathode half cell is
Ag2O (s) +H2O (l) +2e- ---> 2Ag(s) +2OH- (aq); E0 =0.34 V
E0cell = E0cathode -E0anode
= 0.34 -(-0.76) = +1.10 V
Sponsor Area
A device that converts energy of combustion of fuels like hydrogen and methane, directly into electrical energy is known as
-
fuel cell
-
electrolytic cell
-
dynamo
-
Ni-Cd cell
A.
fuel cell
A fuel cell is a device that converts the energy of combustion of fuels like hydrogen and methane, directly into electrical energy.
Electrolytic cell converts electrical energy into chemical energy. Dynamo is an electrical generator that produces direct current with the use of a commutator.
Ni-Cd cell is the type of rechargeable battery which consists of a cadmium anode and a metal grid containing NiO2 acting as a cathode.
A hypothetical electrochemical cell is shown below
A- | A+ (xM)|| B+ (yM)|B+
The emf measured is +0.20 V. The cell reaction is:
-
A+ + B → A + B+
-
A+ + e- → A ; B+ + e- → B-
-
the cell reaction cannot be predicted
-
A+ + B → A+ + B
D.
A+ + B → A+ + B
Electrochemical cell
A0 | A+ (xM) ||B+ yM) B+
The emf of cell is +.20 V. so cell reaction is possible. The half cell reaction are given as follows:
(i) At negative pole:
A → A+ + e- (oxidation)
(ii) At positive pole:
B+ + e- → B (reduction)
A +B- → A+ + B-, Ecello = + 0.20 V
A solution contains Fe2+ Fe3+, and I- ions. This solution was treated with iodine at 35o C.Eo for Fe3+/ Fe2+ is +0.77 V and Eo for I2/ 2 I- = 0.536 V. The favourable redox reaction is
-
I2 will be reduced to I-
-
There will be No redox reaction
-
I- will be oxidised to I2
-
Fe2+ will be oxidised to Fe3+
C.
I- will be oxidised to I2
Al2O3 is reduced by electrolysis at low potentials and high currents. If 4.5 x 104 An of current is passed through molten Al2O3 for 6h, what mass of aluminium is produced? (Assume 100% current efficiency, at.mass of Al = 27 g mol-1)
-
9.0 x 103 g
-
8.1 x 104 g
-
2.4 x 105 g
-
1.3 x 104 g
B.
8.1 x 104 g
Al2O3 ionises as,
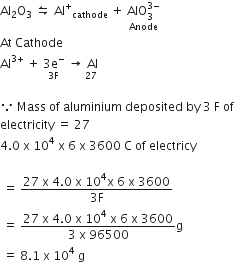
Sponsor Area
Mock Test Series
Mock Test Series





