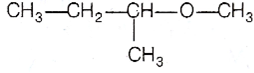Alcohols, Phenols And Ethers
Sponsor Area
Among the following ethers, which one will produce methyl alcohol on treatment with hot concentrated HI?
C.
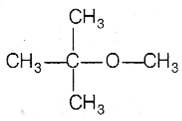
The ether, which gives more stable carbocation, gives CH3OH as one of the product with hot concentrated HI. the order of stability of carbocation is
30 > 20 > 10
Thus, 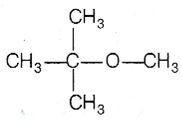 gives CH3 OH as one of the reaction. The reaction proceeds as
gives CH3 OH as one of the reaction. The reaction proceeds as
Sponsor Area
Among the following four compounds
A) Phenol
B) Methyl Phenol
C) meta - nitrophenol
D) para-nitrophenol
the acidity order is
-
D > C > A > B
-
C > D > A> B
-
A > D > C > B
-
B > A > C> D
A.
D > C > A > B
An electron withdrawing group (-I showing group like -NO2, -CN) stabilises the phenoxide ion, thus when present, increases the acidity of phenol. On the other hand, the electron releasing groups (+I showing group like -CH3, -C2H5), when present, decrease the acidity of phenol by destabilising phenoxide ion. Hence, the correct order of acidity of given compound is
p-nitrophenol > m-nitrophenol > phenol > methyl phenol
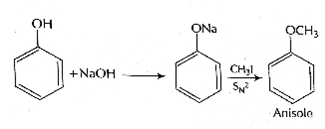
Among the following sets of reactants which one produces anisole?
-
CH3CHO;RMgX
-
C6H5OH;NaOH; CH3I
-
C6H5OH;neutral FeCl3
-
C6H5-CH3;CH3COCl;AlCl3
B.
C6H5OH;NaOH; CH3I
Williamson's synthesis
Consider the following reaction,
the product,Z,is
-
toluene
-
benzaldehyde
-
benzoic acid
-
benzene
C.
benzoic acid
i) Zn dust converts - OH group into - H
ii) Reaction with CH3Cl in presence of anhy. AlCl3 is called Friedel -Craft's alkylation.
iii) Alkaline KMnO4 converts complete carbon chain which is directly attached with the benzene ring, into -COOH group.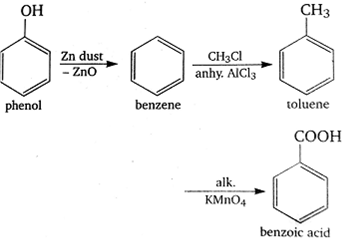
Given are cyclohexanol (I), acetic acid (II), 2,4,6-trinitropenol (III) and phenol (IV). In these, the order of decreasing acidic character will be
-
III > II> IV> I
-
II > III> I > IV
-
II > III> IV> I
-
III > IV> II > I
A.
III > II> IV> I
Higher the tendency to give a proton, higher is the acidic character, and tendency to lose a proton depends upon the stability of intermediate, ie, carbanion formed.
2,4,6 - trinitrophenol after the loss of a proton gives 2,4,6 -trinitrophenoxide ion which is stabilised by resonance -I effect and -M effect thus a most acidic among the given compounds. Phenol after losing a proton form phenoxide ion which is also stabilised by resonance, -M and -I effects but is less stabilised as compared to 2,4,6 trinitrophenoxide ions. Thus, it is less acidic as compared to 2,4,6 trinitrophenol. (CH3COOH) after losing a proton give acetate CH3OO- ion which is stabilised by only resonance. However, it is more resonance stabilised as compared to a phenoxide ion, thus more acidic as compared to phenol. 2,4,6 tri-nitrophenol, however, is more acidic than acetic acid due to the presence of three electron withdrawing - NO2 groups. cyclohexanol gives an anion that is least stable among the given, thus, it is least acidic.
Hence, the correct order of acidic strength is 2,4,6 -trinitrophenol > acetic acid > phenol > cyclohexanol
III> II> IV> I
Sponsor Area
Mock Test Series
Mock Test Series