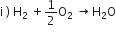1.0 g of magnesium is burnt with 0.56 g of O2 in a closed vessel. Which reactant is left in excess and how much?
-
Mg, 0.16 g
-
O2, 0.16 g
-
Mg, 0.44 g
-
O2, 0.28 g
A.
Mg, 0.16 g
The balanced chemical equation is
Mg + 1/2O2 --> MgO
24g 16 g 40g
From the above equation, it is clear that,
24 f Mg reacts with 16 g O2
thus, 1.0 g Mg reacts with