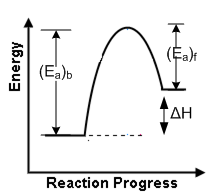
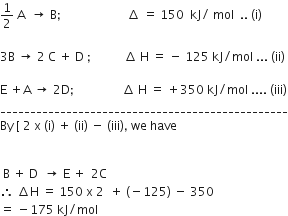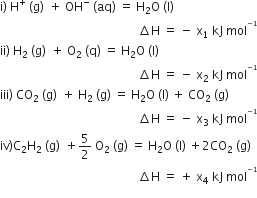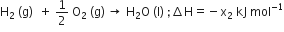A gas is allowed to expand in a well-insulated container against a constant external pressure of 2.5 atm from an initial volume of 2.50 L to a final volume of 4.50 L. The change in internal energy ΔU of the gas in joules will be
-
1136.5 J
-
-500 J
-
-505 J
-
+505 J
C.
-505 J
ΔU = q + w
For adiabatic process, q = 0
∴ ΔU = w
= – P·ΔV
= –2.5 atm × (4.5 – 2.5) L
= –2.5 × 2 L-atm
= –5 × 101.3 J
= –506.5 J
= –505 J