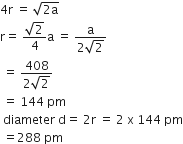A given metal crystallises out with a cubic structure having edge length of 361 pm. If there are four metal atoms in one unit cell, what is the radius of one atom.
-
40 pm
-
127 pm
-
80 pm
-
108 pm
B.
127 pm
Given, edge length = 361 pm
Four metal atoms in one unit cell
i.e effective number in unit cell (z) = 4 (given)
therefore,
It is a FCC structure
Face diagonal = 4r
![]()