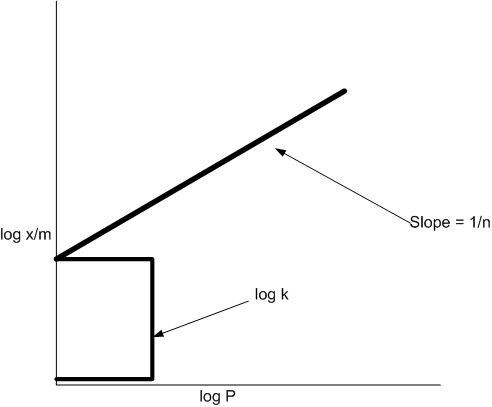
A plot of log x/m versus log p for the adsorption of a gas on a solid gives a straight line with slope equal to:
-
-log k
-
n
-
1/n
-
log k
C.
1/n
The empirical relation x/m = kp1/n put forward by Freundlich is known as Freundlich adsorption isotherm. Taking logarithm
![]()
If the following curve is plotted