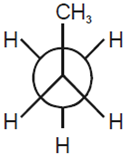
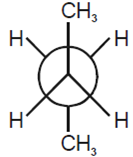
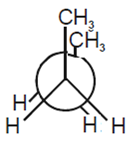
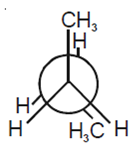
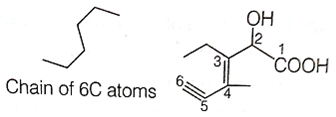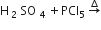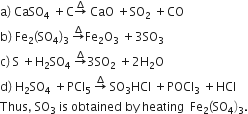Considering the state of hybridization of carbon atoms, find out the molecule among the following which is linear.
-
CH3 - C ≡ C - CH3
-
CH2 = CH - CH2 - C ≡ CH
-
CH3 - CH2 - CH2 - CH3
-
CH3 - CH = CH - CH3
A.
CH3 - C ≡ C - CH3
CH3 - C ≡ C - CH3 linear, because C2 and C3 are sp hybridised.