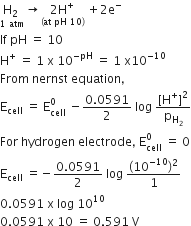A hydrogen gas electrode is made by dipping platinum wire in a solution of HCl of pH =10 and by passing hydrogen gas through the platinum wire at 1 atm pressure. The oxidation potential of electrode would be
-
0.0591 V
-
0.59 V
-
0.118 V
-
1.18 V
B.
0.59 V
For Hydrogen electrode, oxidation half reaction is