"Metals are usually not found as nitrates in their ores"
Out of the following two (a and b) reasons which isare/are true for the above observation?
I. Metal nitrates are highly unstable.
II.Metal nitrates are highly soluble in water.
-
I and II are true
-
I and II are false
-
I is false but II is true
-
I is true but II is false
C.
I is false but II is true
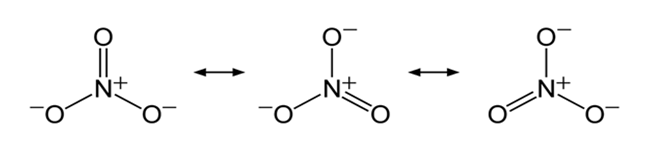
Metals are usually not found as nitrates in their ores, because metal nitrates are highly soluble in water. For example, KNO3 (salt peter) would be classified as completely soluble. Thus, KNO3 could be expected to dissociate completely in aqueous solution into K+ and NO3- ions.
![]()
The nitrate anion has three equivalent oxygen surrounding a central nitrogen atom. This tends to spread the single negative charge and make it easier for water to separate the ions in solution.